
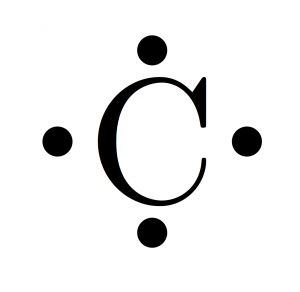
For example, the electron configurations of the first four elements, hydrogen, helium, lithium, and beryllium, look like. The Pauli Exclusion Principle arises from more than just the electrostatic repulsion of negative electrons: it comes from fundamental physical principles that constrain all subatomic particles. 8 Atomic Structure, Electron Configuration, Spectroscopy, Periodic Trends AP. One cool detail that you might notice is that the electron configurations of successive elements (ordered by their periodic number) contain each other. The reason that electrons tend to stay in their separate orbitals rather than piling on top of one another is the Pauli Exclusion Principle, a theorem from quantum mechanics that dictates that no two electrons can ever be in the same place. This results in beautiful geometric structures called orbitals that represent the distinct regions around the nucleus that each electron traces out. While these electrons all stick within the atom because of their attraction to the protons, they also mutually repel each other, causing them to spread out around the nucleus in regular patterns. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons. Therefore carbon atom shares its valence electrons with other atoms of carbon or with atoms of some element forming a Covalent Bond.Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. Since 4 electrons are to be lost, the energy needed for the purpose is going to be very high and not easily available. CC BY-NC 3.0 (opens in new window) Electron configuration (1s2 2s2 2p2) Step 3: Think about your result. 4 extra electrons.Ģ) It could not be lose 4 electrons present in the valence shell to some atom and become C 4+ Carbon has sixelectronsafter filling2 electrons in 1Sand2Sthen the remaining electrons should enter the 2P subshellas it comes next in the order of. Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons. The isotope shown here is carbon-12, with a nucleus of 6 protons (red) and 6 neutrons (blue). It would be difficult for the nucleus with 6 electrons to hold on 10 electrons i.e. In this case the energy required is expected to be very high in order to overcome the repulsion in the electrons already present in the carbon atom and the electrons which it has to accept.
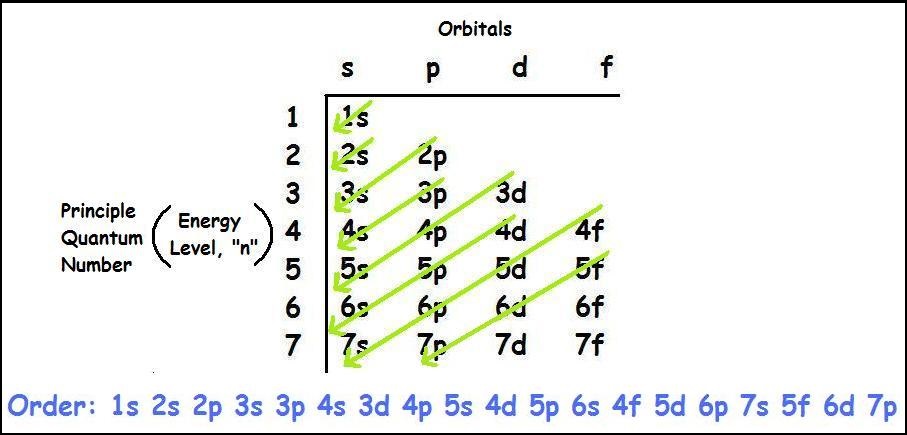
Bonding in Carbon CompoundsĮlectronic configuration of carbon is 2,4.It has 4 electrons in its outermost shell and it can lose or gain four electrons to attain noble gas configuration.ġ) It could not be gain 4 electrons from some other atom and become C 4.
Since it has 4 valence electrons, it is tetravalent in nature.Ĭarbon dioxide gas is 0.03 % by volume of air. Question 7 Why carbon forms covalent compounds? CarbonĬarbon has atomic number 6 and atomic mass 12. carbon electron configuration With four valence electrons, carbon can form four covalent bonds with a variety of atoms. The notation describes the energy levels, orbitals, and the number of electrons in. Question 6 Why is carbon atom tetravalent in nature? A special type of notation is used to write an atoms electron configuration.


 0 kommentar(er)
0 kommentar(er)
